Introduction
Many pts with advanced (adv) stage follicular lymphoma (FL) present with low burden asymptomatic disease and are suitable for observation. Previous studies have shown that rituximab can delay the need for definitive therapy in these pts (Ardeshna et al, Lancet Oncol 2014), and that rituximab maintenance does not offer a long-term advantage (Kahl et al, JCO 2014). Based on early results of these trials, BC Cancer endorsed the use of rituximab monotherapy (R-mono), administered as 4 weekly doses for pts with newly diagnosed asymptomatic adv stage FL in British Columbia in 2011. The aim of this study was to assess the benefit of R-mono and to compare outcomes to pts undergoing observation (OBSE) prior to the introduction of R-mono (OBSE-1) or since the availability of R-mono (OBSE-2).
Methods
The BC Cancer Lymphoid Cancer and Provincial Pharmacy Databases were used to identify all pts >16 y of age with newly diagnosed, adv stage (extensive stage 2-4), asymptomatic (low tumour burden), grades 1-3a FL betw Jan 2011-Aug 2019, who were treated with R-mono. Results were compared with two OBSE cohorts with similar clinical inclusion criteria; a historical cohort (OBSE-1) diagnosed Jan 2004-Dec 2010 and a current-era cohort (OBSE-2) diagnosed Jan 2011-Aug 2019. Use of R-mono was at the discretion of the treating physician and reflected patient preference. The primary endpoint was time-to-new treatment (TTNT), measured from date of diagnosis to start of any therapy following R-mono or OBSE (systemic, radiotherapy or splenectomy); pts who did not need new treatment were censored at last follow-up. Clinical factors were compared using chi-square tests, ANOVA, and Kruskal-Wallis tests. Kaplan-Meier curves were used to generate survival estimates and differences between cohorts were examined using a log-rank test. Multivariable cox proportional hazard models were used to estimate hazard ratios for each cohort adjusting for relevant clinical factors, including the FLIPI group.
Results
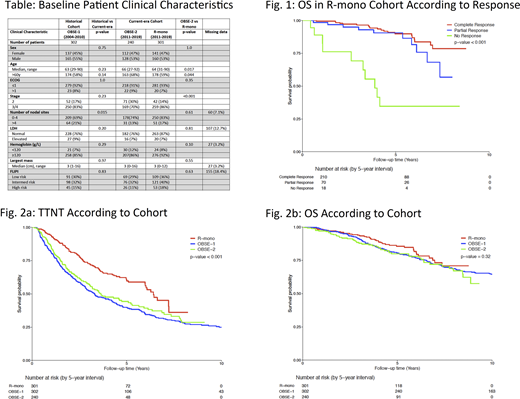
In total, 843 pts were identified; R-mono (n=301), OBSE-1 (n=302), OBSE-2 (n=240). Median f/up was longer for OBSE-1 (12.1y, range 0.2-16.2), compared with R-mono (4.5y, 0.1-8.8) and OBSE-2 (4.3y, 0.4-9.3). Median time from diagnosis to R-mono was 0.21y (range, 0.05-1.06). Clinical characteristics were largely comparable betw the historical (OBSE-1) and current-era cohorts (R-mono + OBSE-2). However, in the current era, pts receiving R-mono were slightly younger and more likely to have stage 3/4 disease than OBSE-2 pts, indicating a possible selection bias in the use of R-mono (Table). Of the 301 pts receiving R-mono, 298 were evaluable for response, with 210 (70%) achieving CR(u), 70 (23%) PR, and 18 with non-response (2 stable disease, 16 progression, 6%). 10/18 R-mono non-responders developed transformed disease, with significantly poorer OS than responders (p<0.001) (Fig. 1). However, time-to-transformation was not significantly different betw the R-mono, OBSE-1 and OBSE-2 cohorts (p=0.40). Overall, TTNT was significantly improved in the R-mono cohort (p<0.001), with 3y and 5y TTNT estimates as follows: 3y TTNT R-mono 73% (95% CI 67-79), OBSE-1 52% (47-58), OBSE-2 57% (51-65); 5y TTNT R-mono 61% (54-68), OBSE-1 40% (35-46), OBSE-2 44% (37-52) (Fig. 2a). Median TTNT was 6.4y, 3.3y and 3.6y in R-mono, OBSE-1 and OBSE-2, respectively. Hemoglobin <120 (p=0.037), number of nodal sites >4 (p<0.0001), and high-risk FLIPI (p< 0.001) were associated with a shorter TTNT. On multivariate analysis controlling for relevant clinical factors and FLIPI, R-mono remained an independent predictor for TTNT (HR 0.53, 95% CI 0.41-0.69, p<0.001). With available f/up, OS was not statistically different (p=0.32). 5y OS was 86% (95% CI 81-91), 81% (76-85) and 80% (74-86) in R-mono, OBSE-1 and OBSE-2, respectively (Fig. 2b).
Conclusions
This is the largest study to date evaluating the benefit of R-mono in pts with newly diagnosed adv stage asymptomatic FL. The use of R-mono administered over 1 month delayed the median time to start of new treatment by approximately 3 years. Non-responders to R-mono had a high rate of transformation and poor OS, and may represent pts with high-risk biology. While the overall rate of transformation and OS were not altered, pts receiving R-mono were approximately 50% less likely to require definitive therapy at 5-years, enabling some pts to avoid treatment toxicity and providing time for novel therapies to emerge.
Perry:Seattle Genetics: Honoraria. Villa:Seattle Genetics: Consultancy, Honoraria; Purdue Pharma: Consultancy, Honoraria; Nano String: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria; AZ: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Sandoz Canada: Consultancy, Honoraria; Immunovaccine: Consultancy, Honoraria. Gerrie:Sandoz: Consultancy; Roche: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Astrazeneca: Consultancy, Research Funding. Scott:Abbvie: Consultancy; AstraZeneca: Consultancy; Celgene: Consultancy; Janssen: Consultancy, Research Funding; NanoString: Patents & Royalties: Named inventor on a patent licensed to NanoString, Research Funding; NIH: Consultancy, Other: Co-inventor on a patent related to the MCL35 assay filed at the National Institutes of Health, United States of America.; Roche/Genentech: Research Funding. Savage:Abbvie: Consultancy, Honoraria; Verastem: Honoraria; Takeda: Honoraria; Servier: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Sehn:AbbVie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Acerta: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Merck: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Teva: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Chugai: Consultancy, Honoraria; Verastem Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.